All Product News
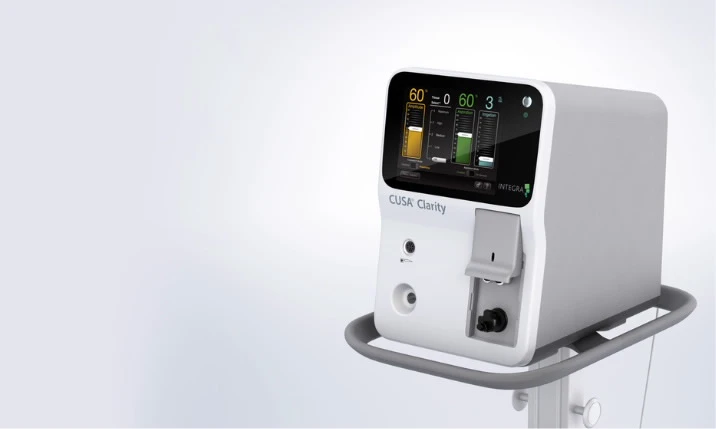
Integra LifeSciences Receives FDA 510(k) Clearance for Use of the CUSA® Clarity Ultrasonic Surgical Aspirator System for Cardiac Surgeries

Integra LifeSciences Announces First Patient Enrolled in Acclarent AERA® Pediatric Registry
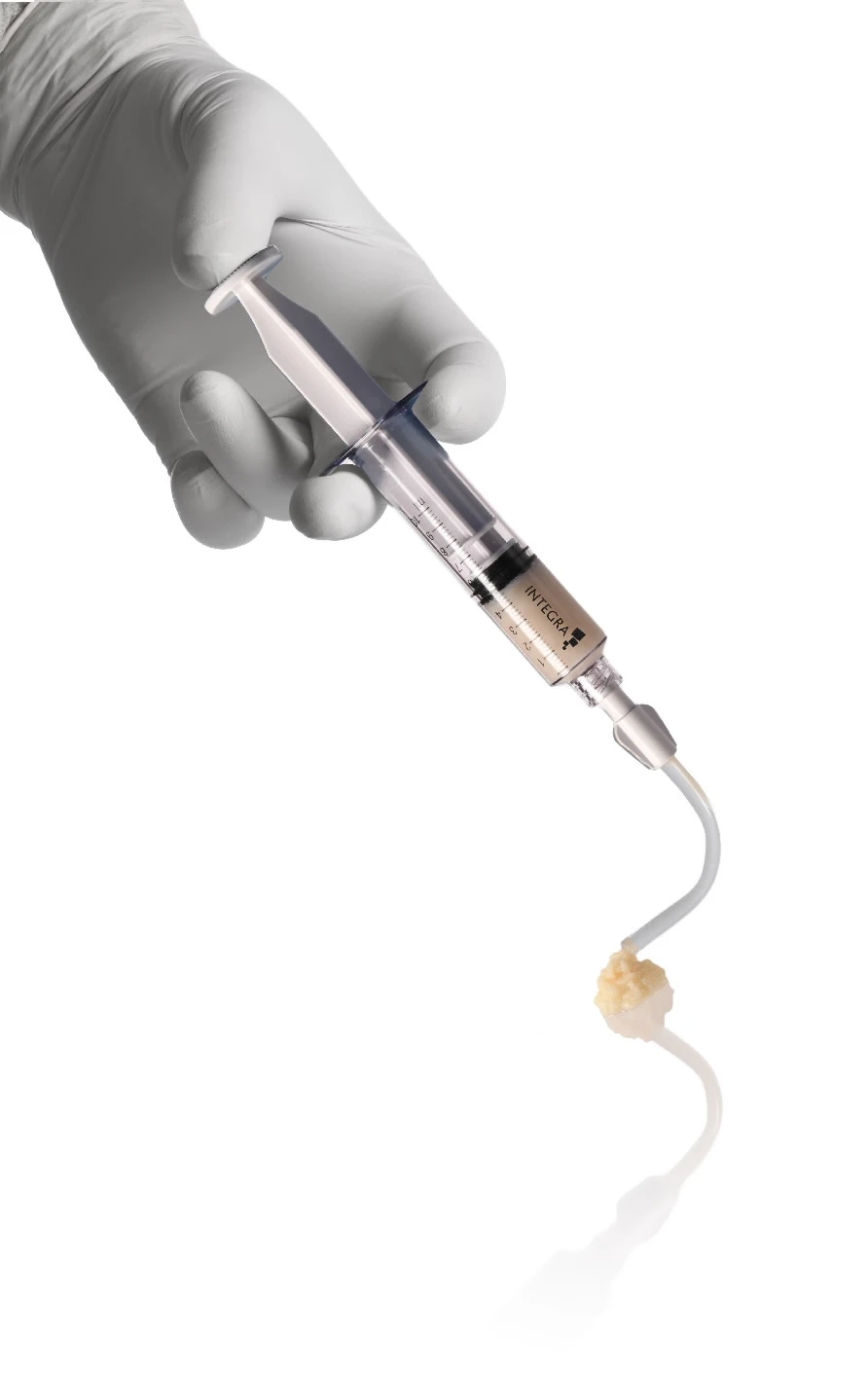
Integra LifeSciences Launches MicroMatrix® Flex To Provide Convenient Access To Hard-To-Reach Areas In Complex Cases
Novel system features flexible tip to deliver flowable version of the Urinary Bladder Matrix (UBM) technology

Integra® Bilayer Wound Matrix (IBWM) 20th Anniversary
On the 20th anniversary of Integra® Bilayer Wound Matrix (IBWM), Integra's John Kemnitzer, senior director, Product Development, shares his history and knowledge about this product and how this technology transformed lives one patient at a time. Watch the video below.

A Lifesaving Collaboration Built on Swiss Watch Precision
Luc Tissot, a visionary keenly attuned to the latest technological advances, created the Tissot Foundation in 1980 with the idea of applying the precision of Swiss watchmaking to other industries such as medical technologies. Coincidentally, in another part of the world, Professor Salomón Hakim, a leading neurosurgeon from Bogotá, Colombia, was looking for a way to perfect a programming system – inspired by watchmaking technologies – to regulate pressure in the brain and better treat hydrocephalus. That’s when he turned to Luc Tissot.

A Look Back: 25-Year History of Integra® Dermal Regeneration Template
This month marks the 25th anniversary of the U.S. Food & Drug Administration (FDA) approval of the Integra® Dermal Regeneration Template (IDRT) for the treatment of life-threatening burns. This regulatory milestone in 1996 – the first approval with a claim of regeneration of dermal tissue – was a pivotal moment for Integra and took more than two decades of research.
Integra LifeSciences Introduces New Codman Specialty Surgical Products At AANS 2019
Integra LifeSciences Holdings Corporation (Nasdaq: IART), a leading global medical technology company, announced today that it will feature its latest innovations at the American Association of Neurological Surgeons (AANS) Annual Scientific Meeting, April 13–17, 2019 in San Diego, California.

Integra LifeSciences Announces The Approval Of DuraGen® In Japan
Integra LifeSciences Holdings Corporation (Nasdaq:IART), a global leader in medical technology, announces the recent approval of DuraGen® Dural Regeneration Matrix in Japan. DuraGen is the first and only non-autologous collagen xenograft approved for use as a dural substitute in Japan.

Research Suggests that Active Leptospermum Honey May Be a Viable Treatment for Partial Thickness Burns
Integra LifeSciences’ MediHoney®, an Active Leptospermum Honey (ALH) dressing for wounds and burns, was the chosen treatment for a prospective assessment of MediHoney conducted by collaborating researchers at West Penn Hospital Burn Center and West Virginia University, Department of Psychology. During the study, seven researchers set out to determine the time-to-heal, patient satisfaction and cost of treatment after applying ALH gel to patients with partial thickness facial burns.

Integra LifeSciences Selected for Healogics iSupply℠ Program
Integra LifeSciences Holdings Corporation (Nasdaq:IART), a leading global medical technology company, today announced it has been selected as a primary provider for cellular-based tissue products within Healogics® Inc. new iSupply program. This program is exclusively available to Healogics’ hospital partners and is focused on ensuring hospitals and patients receive the best value from wound care products commonly used in Wound Care Centers® and other sites of care.

AlloSource and Integra LifeSciences Announce Anchor Gifts to Phoenix Society for Burn Survivors' Never Alone Campaign
AlloSource, one of the nation's largest providers of cellular and tissue allografts, and Integra LifeSciences, a leading global medical technology company, today announced anchor gift donations to the Phoenix Society for Burn Survivors' Never Alone Campaign. The campaign is dedicated to raising money for strategic initiatives that will enable the Phoenix Society to provide a community of support, as well as resources and tools, for burn survivors and their families.

Integra LifeSciences Provides Preliminary Assessment of Minor Damage to its Manufacturing Facility in Añasco, Puerto Rico
Integra LifeSciences Holdings Corporation (NASDAQ:IART), a leading global medical technology company, today announced that its manufacturing facility in Añasco, Puerto Rico sustained relatively minor damage from the impact of Hurricane Maria. A team is on-site to oversee repairs.